Cardiovascular Disease High Sensitive Troponin I
본문
High Sensitive Troponin I
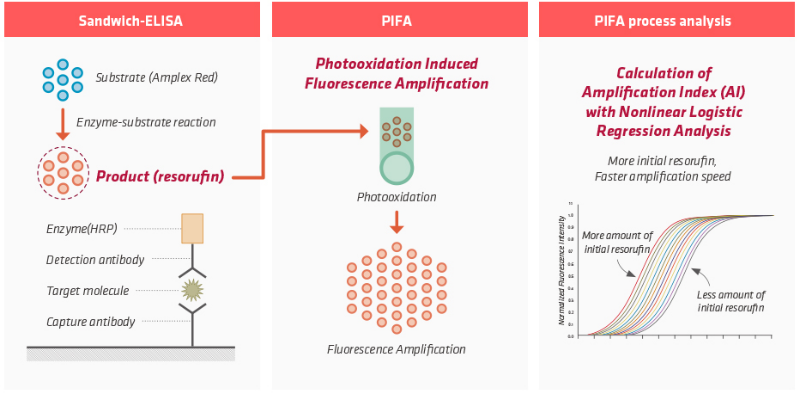
• Absoludy Ultra Troponin I is PIFA Technology(Photooxidation induced Fluorescence Amplification) based quantitative measuring diagnostic test.
• Tnl-Ultra is for detecting myocardial infarction (MI) and assessing risk of adverse events in patients presenting with ischemic symptoms suggestive of acute coronary syndrome. [NCBI]
• Measuring the level of Ultra Troponin I To exclude AMI(Acute myocardial infarction) from other myocardial injury particularly suitable for emergency testing.
Anti-cancer therapy monitoring
• Absoludy Ultra Troponin I is PIFA Technology(Photooxidation induced Fluorescence Amplification) based quantitative measuring diagnostic test.
• Measuring the level of Ultra Troponin I to help monitoring of chemotherapy related cardiotoxicity which may allow for earlier realization of the degree of cardiac damage occurring during treatment, creating the opportunity for more timely modulation of therapy.
• Cardiotoxicity is a common complication that may compromise the clinical effectiveness of anticancer therapy, impacting both cardiologic and oncologic patients' outcome. The early identification, prevention and treatment of cardiotoxicity remain an important strategy to reduce morbidity and mortality in cancer patients.
|
AMR |
10~15,000 pg/mL |
|
Test time |
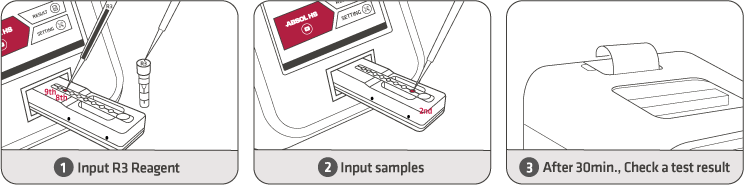
30 Min |
|
Samples Type |
Serum and Plasma (Lithiumheparin and K₂-EDTA) |
|
Samples Volume |
20 uL |
|
Contact |
Cartridge_10EA |
|
Pretreatment_20EA |
|
|
Disposable Tip_24EA |
|
|
Code Chip_1EA |
|
|
Package Insert_1EA |